Temperature pressure and volume c. Ideal gas law b.
Temperature and pressure only b.
. D Volume Temperature Pressure. Volume Temperature Pressure. Therefore the number of moles 12516 39 mol.
These laws relate one thermodynamic variable to another holding everything else constant. The combined gas law relates the variables pressure temperature and volume whereas the ideal gas law relates these three including the number of moles. Join Telegram Group Answer This Question.
Boyles law relates the pressure of gas to its. This problem has been solved. One of the following is not dealt with in the ideal gas law.
The combined gas law relates which of the following a. From PV nRT ideal gas law R constant if n 1. Option D is the correct answer.
Course Title BIOLOGY 121. It is known that one mole any gas contains 6022 10 23 gas molecules. Combined Gas Law Formula.
Combined gas law can be mathematically expressed. It is known from the Avogadros law that at constant temperature and pressure equal volume of all gases contains equal number of moles. Chemistry 19122019 1831 hilljade45 The combined gas law relates which of the following.
There are a couple of common equations for writing the combined gas law. The Combined Gas Law. The combined gas law relates which of the following a pressure and volume only c from BIO 1408-001 at Bright Career College Mailsi.
The Combined Gas Law relates the pressure volume and temperature of a gas to each other. What is the new volume. See the answer See the answer done loading.
Collision between fast neutrons and nitrogen nuclei present in the atmosphere. It is simply a combination of the other gas laws that works when everything except temperature pressure and volume are held constant. Therefore we will use the following form of the combined gas law.
Reffering to its definition the combined gas law relates. A 500 L air sample at a temperature of. Since we have the mass of O2 we can calculate the moles as moles massRMM where RMM is the relative molecular mass of O2 which is 32 gmol.
Action of ultraviolet light from the sun on atmospheric oxygen. Where PVT represent the same variables as in the combined gas law17 мая 2014 г. Group of answer choices.
Which of the following is a correct equation for the combined gas law. Therefore the combined gas law is. The combined gas law relates pressure temperature and volume when everything else is held constant mainly the moles of gas n.
The classic law relates Boyles law and Charles law to state. Therefore both carbon dioxide and hydrogen has equal number of moles at 31 L of volume. The combined gas law relates which of the following.
Pressure and volume only b temperature and pressure only c. Pressure and volume are inversely related to. Atroni 7 5 months ago.
Let us assume that n 1. Charles Law relates the volume of a gas to its. The combined gas law states that the ratio between the pressure-volume product and the temperature of a system remains constant.
The balanced reaction should be 2HgO -- 2Hg O2 therefore 2 moles of mercury are produced for every one mole of oxygen gas. The interdependence of these variables represents combined gas law which states that the ratio between the product of pressure-volume and temperature of a system remains constant. The combined gas law relates which of the following a.
The combined gas law relates which of the following a temperature and pressure from BIOLOGY 121 at Coral Reef Senior High School. The ideal gas law or equation can also be reduced to the general gas law or the combined gas law. The most common form of the equation for the combined gas law is as follows.
Where P pressure V volume T absolute temperature Kelvin. Hence it depends on all the three Volume Temperature Pressure. A its volume increases more under pressure than an equal volume of solid does b The space between gas particles is much less than the space between liquid or solid particles c The volume of a gass particles is small compared to the overall volume of the gas d.
The combined gas law allows you to derive any of the relationships needed by combining all of the changeable pieces in the ideal gas law. The combined gas law expresses the. Lightning discharge in atmosphere.
K is a constant. Action of solar radiations particularly cosmic rays on carbon dioxide present in the atmosphere. The number of moles of an unknown gas can be calculated using.
By changing the volume the pressure of the gas increases to 605 kPa as the temperature is raised to 398 K. School Bright Career College Mailsi. Combined gas law c.
Namely pressure temperature and volume. AIEEE Bank Exams CAT GATE. Gas equation stands for.
School Coral Reef Senior High School. Hence d is correct answer. Volume and temperature only d.
A gas at 155 kPa and 298 K occupies a container with an initial volume of100 L. More than one correct response. The combined gas laws relates which of the following.
The first step is to determine the variables. The combined gas law relates which of the following. Why is a gas easier to compress than a liquid or a solid.
Temperature pressure and volume 1 See answer Advertisement.
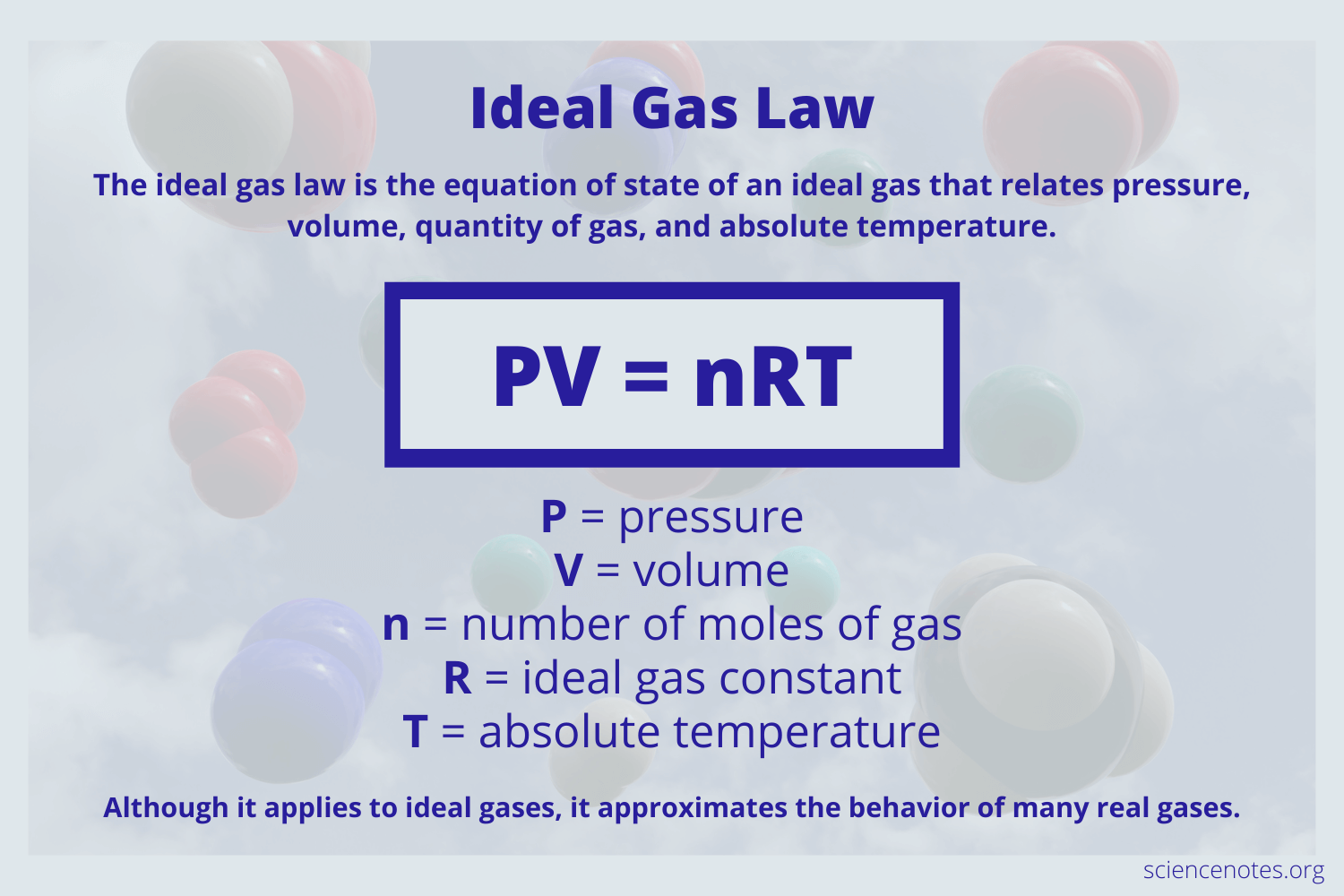
Ideal Gas Law Study Guide Inspirit

0 Comments